Canada is immersed in an opioid crisis. In 2019, 10 Canadians per day died due to opioid overdose. Worryingly, these trends appear to be exacerbated by the COVID-19 pandemic. Since March 2020, rates of fatal opioid overdose have doubled in many parts of the country. Unfortunately, existing strategies to manage opioid addiction are minimally effective. Some 65% of those seeking treatment for opioid addiction will relapse within a month. This is especially dangerous since the risk of death is higher when opioids are used after a period of abstinence. Novel, effective strategies that reduce the likelihood of relapse are needed to improve health outcomes for those suffering from opioid addiction.
Brexpiprazole is an atypical antipsychotic drug currently approved for the treatment of schizophrenia. It acts primarily as a partial agonist at the dopamine receptor. Partial agonists are interesting drugs. Agonists are drugs that bind and activate a specific receptors (antagonists are drugs and block receptors from being activated). Full agonists, like the neurotransmitter dopamine, bind to the dopamine receptor and cause full activation of that receptor. Brexpiprazole, on the other hand, is a partial agonist. That means when it binds to the dopamine receptor, it can only ever partially activate that receptor – even at the highest doses of the drug. When brexpiprazole is alone, it is free to bind the dopamine receptors, but because it only ever partially activates the dopamine receptors, it causes a mild activation of the dopamine system (a ‘tickle’, if you will). This can be advantageous, because while low dopamine levels are associated with depression and loss of pleasure, too much dopamine signalling is associated with psychosis (like in diseases, such as schizophrenia). Brexpiprazole is pretty good at stabilizing the dopamine system, without unwanted side effects, like hallucinations and psychosis. The interesting thing about partial agonists, however, is that they can act as antagonists when in the presence of a full agonist (like dopamine). This is because if brexpiprazole and dopamine are both present, they are competing for binding at the same receptor (those dopamine receptors). If brexpiprazole is bound to a dopamine receptor, dopamine cannot bind and fully activate the receptor. Therefore, brexpiprazole will reduce (or antagonize) the effects of dopamine.
Dopamine signalling in the brain has been tied to many aspects of opioid addiction. For example, opioids, like heroin or fentanyl, cause a dramatic rise in dopamine levels in the brain. This rise in dopamine contributes to the reinforcing or ‘addicting’ properties of drugs, such as opioids. Chronic opioid use, on the other hand, leads to a paradoxical drop in resting dopamine levels (in the absence of drugs of abuse). This ‘hypo’ dopamine state is tied to negative feelings and anhedonia that contribute to the unpleasant constellation of symptoms associated with opioid withdrawal. Brexpiprazole, being a partial agonist at the dopamine receptor, can theoretically improve both of these aspects in opioid dependence. It will reduce the effects of drug-evoked dopamine release. But, it will also gently activate the dopamine system when dopamine levels are low (like during withdrawal).

We tested this hypothesis in our animal model of opioid dependence. Male and female adult mice were treated twice daily with escalating doses of the opioid, morphine. This treatment causes the classical symptoms of opioid dependence, such as analgesic tolerance. It also leads to withdrawal when the drug treatment stops. We treated morphine dependent mice with or without brexpiprazole. We found that brexpiprazole was effective at blocking the rewarding effects of morphine. It also attenuated relapse behaviour during a forced abstinence period and improved some symptoms of opioid withdrawal, including hyperalgesia (increased pain). These results indicate that brexpiprazole is effective at mitigating some of the dopamine-dependent behaviours associated with chronic opioid use. It provides strong preclinical evidence that this drug will be effective at promoting abstinence rates in those seeking treatment for opioid use disorder.
This paper was published in the journal Neuropharmacology in April of this year. The project was led by our PhD Student, Julia Nickols and in collaboration with Dr. Serdar Dursun, Professor and Psychiatrist at the University of Alberta. This work was funded by the Canadian Research Initiative on Substance Misuse (CRISM).
You can find a link to the paper here or download it below.



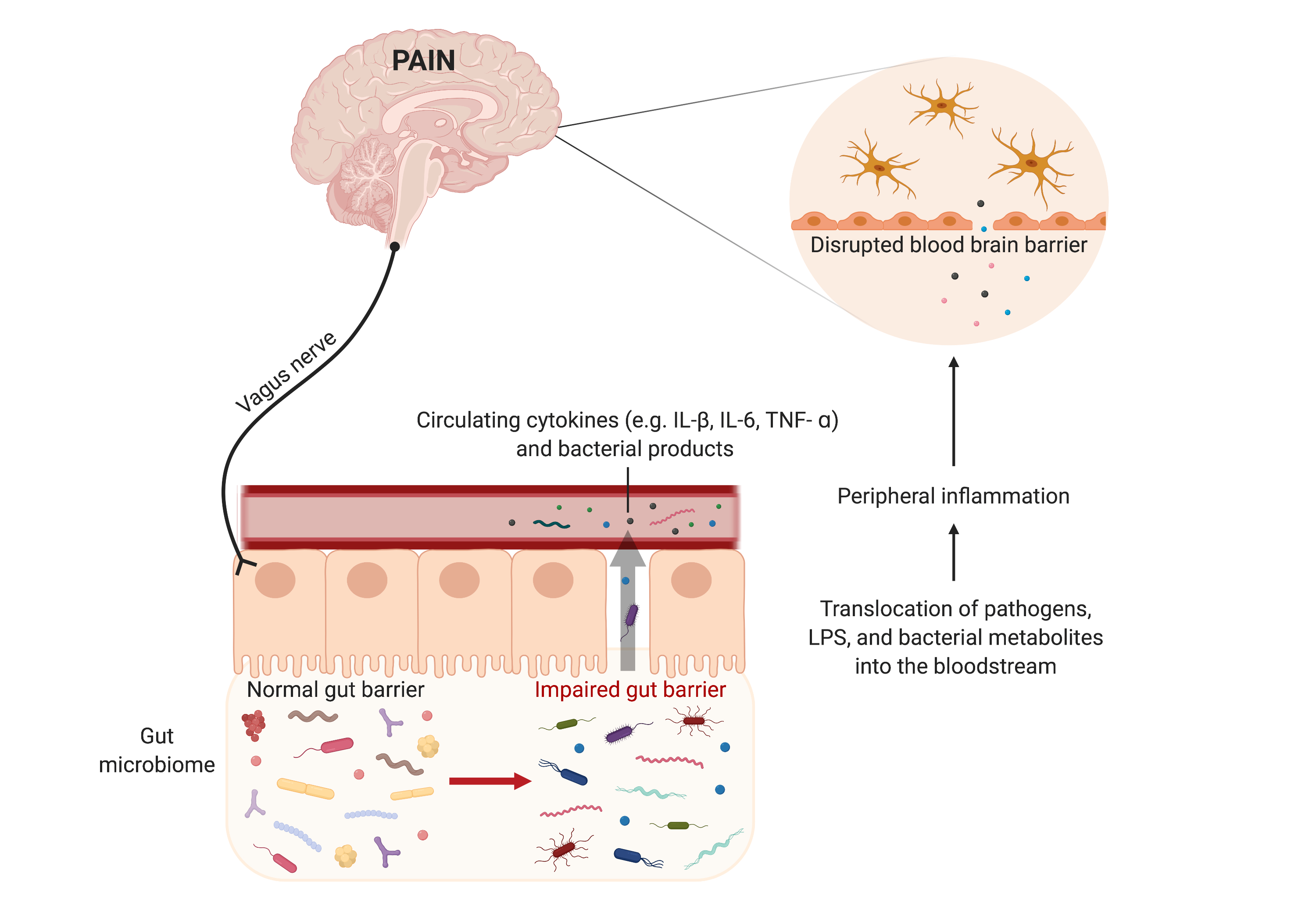

 Dr. Taylor discusses her work on opioids and the gut microbiome (
Dr. Taylor discusses her work on opioids and the gut microbiome (

 The Taylor lab was recently awarded a
The Taylor lab was recently awarded a  We wrote a commentary on the recent paper looking at learned conditioned pain sensitivity in male and females (
We wrote a commentary on the recent paper looking at learned conditioned pain sensitivity in male and females (